
A BREAST CANCER BLOOD TEST
The Test
HERTEST® is the first Martell test available at point-of-care.
Small amounts of human epidermal growth factor receptor 2 (HER2/neu) are shed into the bloodstream and can be detected by an ELISA (enzyme-linked immuonosorbent assay) immunoassay. HERTEST® provides a new look at HER2/neu blood testing using state-of-the-art recombinant rabbit monoclonal antibodies. This highly sensitive test requires only a small amount of blood. That blood is processed (serum separated from blood cells) by your hospital's clinic and then sent to our lab where HERTEST® can be performed. Patient result's are then submitted to your oncologist in as little as 24 hrs.
Branded HERTEST®, our blood test can quickly and inexpensively measure drug efficacy or detect recurrence and new HER2 tumors in women with metastatic breast cancer. HERTEST® monitoring can be compared to the use of blood PSA when used to monitor men with prostate cancer.

Clinical Background
HER2/neu (also known as ErbB2) is a transmembrane receptor protein with an extracellular domain (ECD) and intracellular tyrosine kinase activity. Potentially 50,000 HER2/neu molecules may be present on a single cancer cell, when overexpressed or amplified. These molecules then associate and activate cell growth.
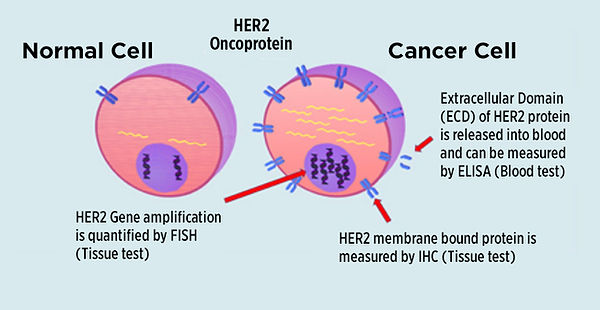
Approximately 20% of invasive primary breast cancers and a significantly higher proportion of metastatic tumors over-express HER2/neu enough to “drive” the cancer. HERTEST® results with levels of sHER2 (serum HER2) beyond an upper limit of normal indicate the presence of a HER2 tumor—false positive results are very rare. A rising sHER2 concentration may be an indicator of recurrence, preceding clinical or radiological evidence by months. Elevated HERTEST® values in the absence of a positive tissue test may signal a role for targeted therapeutics in refractory (cancer not responding to current treatment) breast cancer despite an earlier negative tissue test.
Given the high cost and limitations of targeted therapy, oncologists are seeking ways to reduce drug use yet improve outcomes and the quality of life. HERTEST® monitoring provides useful information to support cancer treatment de-escalation.
Clinical Utility
Immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) provides a measurement of a patient's HER2 status at initial diagnosis of breast cancer. HERTEST complements tissue tests when a tumor spreads by monitoring response to therapy or disease progression. Clinical data indicates that serum levels of HER2 reflect disease progression and response to therapy and that serial monitoring of serum HER2 may be a valuable tool in creating a more efficient treatment regimen. HERSTEST offers the following advantages:
-
HERTEST® is minimally invasive (requiring only a small amount of blood)
-
HERTEST® can monitor metastatic breast cancer patients receiving most common therapies
-
HERTEST® was developed specifically to follow metastatic breast cancer patients to help determine any change in serum HER2 status
-
HERTEST® simplifies routine monitoring of metastatic breast patients with elevated serum HER2
To be of maximum value, blood (serum) tests should fulfill four specific criteria—HERTEST® scores high in all four measures.
-
PROGNOSTIC—A high serum HER2 correlates with a smaller likelihood of pathological complete response and with a shorter disease-free survival.
-
PREDICTIVE—An elevated serum HER2 predicts response to targeted and cytotoxic drugs.
-
Serum HER2 is UNRELATED TO TUMOR SIZE.
-
Serum HER2 must be considered INDEPENDENTLY OF TISSUE HER2—Tissue negative primary tumors give rise to HER2 positive metastases, often called a phenotypic shift.
.png)
Free of Recurrence Recurrence Poor Prognosis
Each of the above criteria has been studied extensively. Reports from Germany, Spain, France, the U.S., and elsewhere corroborate monitoring in over 10,000 breast cancer patients.

